Section B: Rehab after fracture
Initiation and progression of resistance
Flowers et al. (2022) review is currently the only study combining clinical and physiotherapy setting. They wanted to answer the question: when to initiate and when to progress resistance training after fracture.
Current research on progressive weight-bearing for fractures is limited. While some studies suggest that early, progressive weight-bearing can lead to good short-term functional outcomes and low complication rates, the overall evidence for long-term outcomes (quality of life, return to activity) is weak.
Few studies clearly define how weight-bearing should be advanced. Variations in fracture type and hardware fixation further complicate standardization.
Most studies rely on "weight bearing as tolerated," with pain as the main guide, rather than using tissue-healing-based criteria.
Due to the lack of clear progressive weight-bearing protocols in the existing literature, definitive clinical recommendations can not be made.
"although detailed orthopedic management strategies are well defned, rehabilitative interventions, in terms of type, intensity, frequency, and timing, are not adequately described and specifc recommendations for the assessment of patients’ functioning are lacking" (Gimigliano et al., 2020)
Ankle fractures
he traditional weight-bearing protocol involves 10 to 12 weeks of non-weight bearing, followed by a 4 to 6-week period of progressive weight bearing. During the first week of this phase, patients bear 25% of their body weight, increasing by 25% each subsequent week until full weight bearing is achieved. Alternatively, initiating rehabilitation as early as postoperative day 2—with early mobilization and weight bearing—may provide better outcomes in terms of pain management and recovery. Starting rehabilitation interventions within 10 days after surgery may also help reduce postoperative complications (Zhao et al., 2022).
But concrete conclusions and guidelines can not be drawn. Appropriate rehabilitation time and protocol should be selected according to the patient.
Sternotomy
In the systemic review and meta-analysis by J. Pengelly et al. (2019) the outcomes indicate that there is no consistency in how resistance training is applied, progressed and evaluated following median sternotomy. There is an urgent need to investigate the effects and optimal timing of weighted upper limb and trunk resistance exercises on functional, cognitive and physical recovery.
The development of sternal precautions was based on the belief that arm movements during the first 1–6 weeks after surgery could increase stress on sternal wires and skin, risking sternal separation. Early research suggested chest muscle activity could cause bone edge movement over 2.0 mm, threatening bone healing. However, recent studies have shown that gentle upper limb movements during this period do not harm sternal healing, reduce pain, and cause less than 2.0 mm of micromotion. Interestingly, coughing causes the most sternal separation post-surgery (Pengelly et al., 2022; Rauschenberg et al., 2020).
At 2 weeks after surgery, participants in the study by J. Pengelly et al. (2022) performed six upper limb exercises using a 20-lb weight for 10 reps. The biggest increases in average weight lifted occurred with the triceps dip at 8 weeks (81.1 lb) and the shoulder pulldown at 14 weeks (90.9 lb), both done for 12 reps. This shows that we can challenge our patients following sternotomy.
Progression: If the patient does not feel discomfort or pain the weigth is progressed by 5–10% of the initial weight until patients are able to exercise within the desired range (RPE 13/20 or volitional fatigue after 9–12 repetitions) (J. M. S. Pengelly et al., 2020).
Assist fracture healing
The morbidity and socioeconomic costs of fractures are considerable. The length of time to healing is an important factor in determining a person's recovery after a fracture. Are there clinically relevant interventions to promote bone healing after fracture?
Whole body vibration: the narrative is based on tissue healing via promoting angiogenesis in order to enhance bone repair. Current evidence indicate a lack of standardized clinical trials for the treatment of fractures in humans. The use of WBH seems safe to use but there are no protocols. But overall it is too early to say that WBV can assist bone healing (Wang et al., 2017; Ganse, 2024).
Ultrasound and shock wave: Pain scores and patient‐reported outcome measures (PROMS) were unlikely to be clinically important, and the certainty of the evidence was very low in overall. Generally there were no treatment‐related adverse events (Searle et al., 2023).
Fracture rehab for kids and adolescent
Gimigliano et al. (2021) described the current research gap in pediatric population, phasing the same problems as with adults. Clinical management seems to be good established but post-operative rehabilitation with proper load management is lacking fundamental research.
There is limited information available on therapeutic exercises to be performed during or after the use of orthoses. One possible reason for the scarcity of rehabilitative recommendations for children and adolescents following fractures is the high rate of spontaneous bone healing typically observed in this population. Additionally, joint stiffness and other complications are rarely reported in pediatric patients compared to adults, making physiotherapy seem unnecessary.
Age on fracture healing times
Although fracture healing tends to be slower and less predictable in older adults, clinical study findings on age as a risk factor for fracture nonunion remain inconsistent. Comorbidities such as smoking, obesity, and diabetes likely confound these results, making it difficult to determine the independent impact of age. Therefore, future research should be carefully designed to distinguish the effects of aging from those of associated health conditions (Saul & Khosla, 2022).
Effect of osteoporosis on fracture healing
Does osteoporosis delay or impair healing in fractured bones? since the biological factors that contribute to bone remodelling are affected by osteoporosis.
It seems that callus formation takes longer, but does not effect callus strength. (Giannoudis et al., 2007).
Though it appears intuitive that osteoporosis is likely to have a significant impact on fracture healing, obtaining definitive evidence to prove this assumption has been difficult for many methodological reasons, for example the definition of healing as most studies have considered the formation of bony callus with bridging as the end point of fracture healing, whereas evaluation of the subsequent remodelling phase has rarely been performed. So in summary, clinical studies investigating the potential association between osteoporosis and delayed fracture healing or non-union are overall insufficient in providing clear evidence (Chandran et al., 2024).
Effect of osteoporosis medications on fracture healing
Anti-resorptive agents inhibit the activity of osteoclasts, but given that osteoclastic bone resorption is an important step in fracture repair, the question wether osteoporotic medication (especially bisphosphonates) is justified in treatment for fractures in osteoporotic population.
Xue et al. (2014) and Palui et al. (2022) provide reassurance that bisphosphonates are safe to use following an acute fracture, as no significant delay in fracture healing has been reported in most of the studies, although some heterogeneity existed amongst the studies.
But nevertheless the efficacy of bisphosphonates in preventing secondary fractures outweighs the miniscule potential risk of delayed or non-union of fractures that may occur (Chandran et al., 2024).
Fracture reinjury risk in sports
Fractures account for 5-10% of all sports injuries, with long RTS timelines.
The first question, when looking at fracture management needs to be, whether it's going to be conservative or surgical. The majority of upper extremity sport fractures are (surprisingly) treated conservatively (around 90% of them).
When it comes to high level athletes, decision making concerning conservative vs. surgical does always focus on the proposed time for RTS. And quite often, the surgical procedure is chosen as a safe route, in order to prevent time loss from an unsuccessful attempt at conservative therapy (which might be the fist line treatment in the non-athletic population). Even surgical techniques are chosen depending on that aspect.
RTS from a fracture (at least regarding contactless RTS) can take anywhere from 2 weeks (finger phalanx fractures) up to 12+ weeks (tibial shaft fractures) depending in site and severity of injury (Robertson & Wood, 2015). Radiological union in usually warranted for the more complicated fractures, to permit RTS (with full contact).
A retrospective review performed in the professional soccer leagues found that after lower extremity fracture (femur, tibia or fibula) 80% of players did return to play (RTP). With a concerning 12% overall reinjury risk (but only a 4% risk of refracture of the same bone). The good thing, as the authors stated, is that even with increased risk of reinjury in elite soccer players "career longevity and performance is not compromised" (Lavoie-Gagne et al., 2021).
While the return to play rate seems encouraging, it was associated with a mean absence form the field of 5 months. The highest incidence of fracture was found in the fibula, closely followed by the femur and then tibia.
Return to play rates unsurprisingly vary with the place of fracture. As another study, again looking at professional athletes, found a RTP rate of 98.7% after foot surgery (mostly 5th metatarsal and navicular). With athletes reaching their preoperative performance levels within 1 season after surgery (mean of 4-5 months). Interestingly though, the authors found that "the number of games started 1 season after primary surgery is a predictor of reinjury. Players who started with "more games the season after initial surgery were more likely to experience refracture, which generally occurred within 1 year of returning to play" (Singh et al., 2018), suggesting that return to sports should be gradual, and re-injuries happen, even at times where bone should already be "healed"(=several months after first injury).
Risk of reinjury also depends on the sports an athlete is returning to. NBA athletes have higher reinjury risk of foot fractures than other athletes (Singh et al., 2018).
So: is re-fracture risk something to worry about?
Generally, refracture risk in athletes is rare and should be preventable with a well progressed loading regime (e.g. ACUTE:CHRONIC workload ratio). Although bone remodelling is a process that can take years, bones should be sufficiently stable after the usual 6-8 weeks of healing.
Early versus Delayed weight-bearing
The authors of a 2022 systematic review on weight bearing after lower extremity fracture found that "early, progressive weight bearing appears safe for patients following traumatic fracture", but "the literature that does utilize progressive early weight bearing provides little information that is helpful for enhancing the rehabilitation professional’s clinical decision-making".
Weight bearing decision making remains a speculative task, as "conclusive clinical recommendations are not possible at this point" (Flowers et al., 2022).
A recent study compared early (after 2 weeks) vs late (after 6 weeks) weight bearing after ankle fracture surgery. They did not find significant differences between groups at the 4 month follow up, with better cost-efficiency for the early weight bearing group. They stated that "the advantage gained from early weight-bearing is most likely tied to the recognised issues associated with immobility, encompassing stiffness and muscle atrophy, which tends to recover more slowly than it is lost" (Bretherton et al., 2024). This should provide clinicians with information that early weight bearing should be the recommended strategy after ankle fractures.
In the frail population, fracture location has the largest predictive impact on the weight bearing decision (hip fracture vs no hip fracture; with hip fractures allowed to weight bear immediately most of the time, with other locations only 1/3 as much) (Richardson et al., 2022). With early weight bearing being of utmost importance in this population, as "early weight-bearing after surgical care of hip fracture seems to decrease morbidity and mortality" (Warren et al., 2019).
As other single studies propose effectiveness (even superiority) of immediate weight bearing, such as after plate fixation of tibial plateau fractures (Williamson et al., 2018) or intramedullary fixation of distal tibial fractures (Weng et al., 2020), it remains a case-by-case decision making process, as too many factors exist that influence the bone healing timeline (fracture type, place, severity; inflammatory conditions; injured bone; site of injured bone; age; lifestyle factors...) as well as the mechanical stability of the injury (type of surgical or conservative management) that all influence decision making.
Another (unorthodox) point of view:
In general after a fracture, the surgeon will prescribe a period of unloading (non-weightbearing) to allow for a healing environment, without risking implant failure. As too much loading too soon can lead to the development of pseudarthrosis (development of a false joint; after a secondary bone healing response).
This way of practice has gotten some pushback, as there seems to be an optimal window of loading, to increase the callus formation, during the proliferation phase (day 5-21). Even though traditionally "postoperative weight bearing is encouraged only once callus formation can be visualized (consolidation phase) which may be the time when fracture motion should be limited to support bridging". This means that loading should be restricted in the consolidation phase, as "mechanical stimulation is not required at all during the consolidation phase" to regain fracture stiffness (Barcik & Epari, 2021). While this sounds plausible in theory, studies to back up those claim are lacking in humans but if true, might be revolutionary in the future management of fractures.
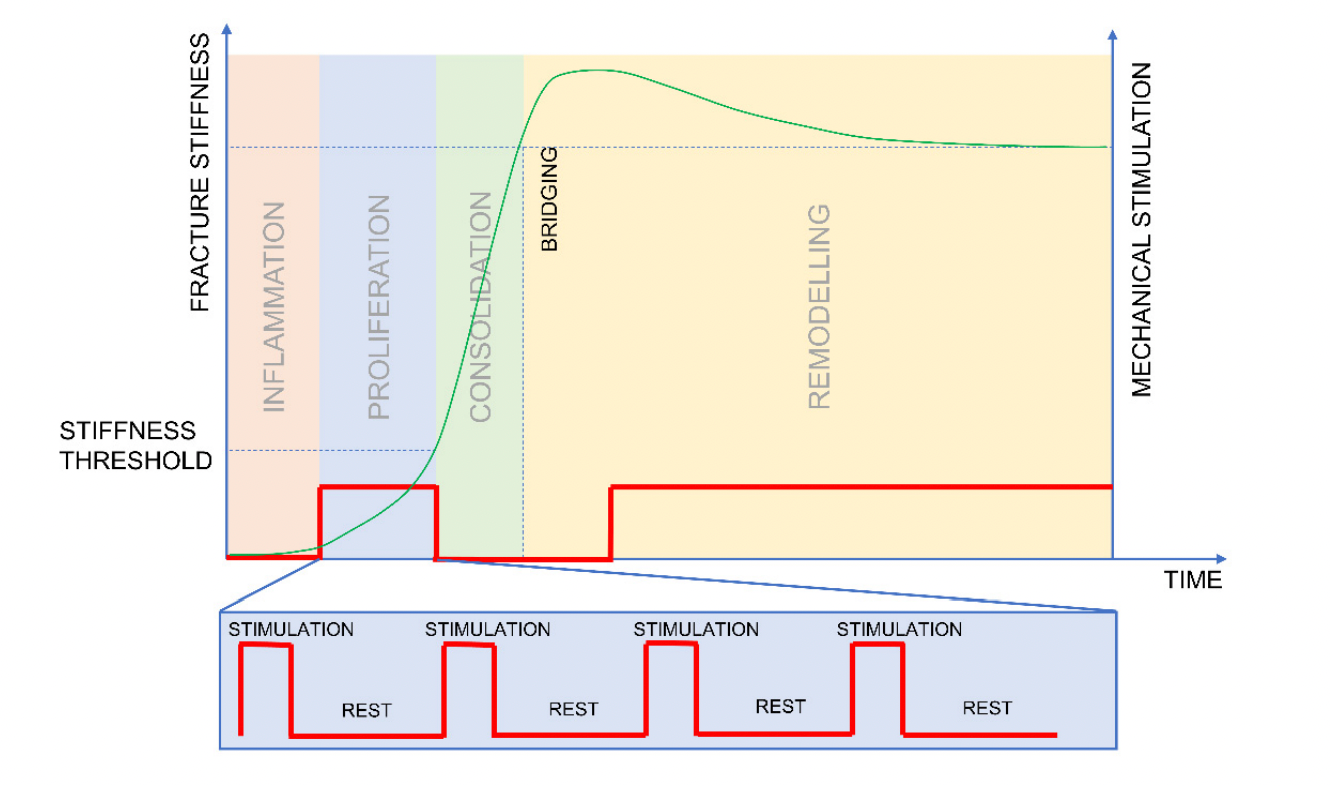
When can we start loading a fractured bone?
We know that different phases of bone healing (inflammatory response, soft callus formation, hard callus formation and bone remodelling) supply the bone with different levels of rigidity and elasticity (in the beginning, granulation tissue can withstanding tensile forces of 0.1 nm/mm2 up to the 130 nm/mm2 of compact bone) (Bigham‐Sadegh & Oryan, 2014). The problem is we have a hard time figuring out when the exact time point is where the bone has had enough healing happening to benefit from progressive weight bearing / exercise protocols.
Contrary to expectations, the injury time-points of refractures are often seen several months into rehab or in athletes when they already returned to sports, with rare incidences of early re-fracture.
The decision of whether an individual is allowed to weight bear ultimately comes down to the operating surgeon. And factors such as pre-injury mobility levels are important (as the more mobile people have higher chances of getting delayed weight bearing instructions as the more immobile people are more often allowed to immediately weight bear).
The below picture shows the Mechanostat Theory, which describes a continuum of mechanical loading. Where too little loading leads to bone loss (catabolic), high enough loading leads to bone synthesis (anabolic) and too high forces lead to bone damage (e.g. fractures).
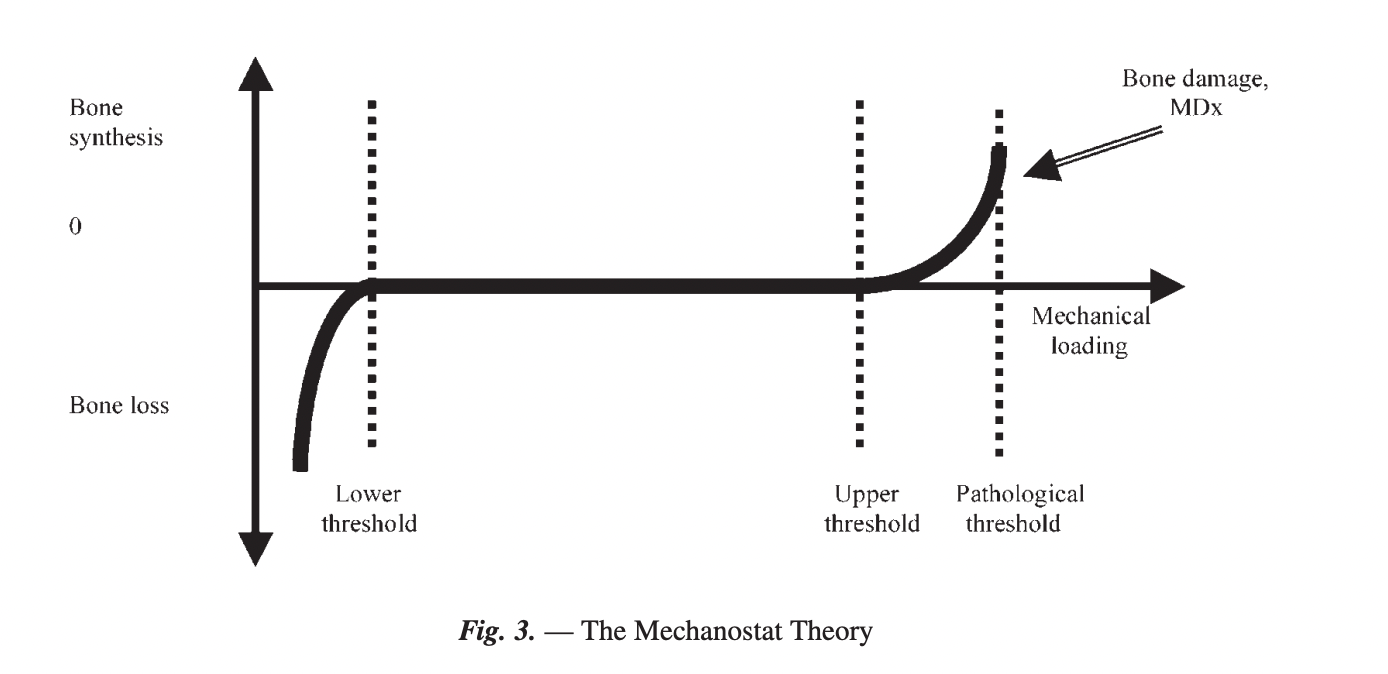
After sustaining a fracture, the space between "Upper threshold" and "Pathological threshold" is markedly reduced. This means, that in a rehabilitative setting, the optimal load needs to be found in order to drive adaptation without including bone damage. It's as simple as that.
While during the early weeks after a fracture, the focus will be on improving a patients joint mobility and getting some muscle activation, the loads on the bone will increase after the fracture has fully healed (after 6-8 weeks). It is wise to stick to completely pain-free activity during these first weeks. Afterwards, bone loading can be progressively increased, with the allowance of some pain. Sadly, no articles could be found in the literature to guide these treatment decisions after fracture, so we rely on the data from a connected field (bone stress injuries), where high risk bone stress injuries are always loaded in a pain free range, until healing as visible on X-ray. Therefore, this is the recommended strategy, with the exception of x-ray, where 6-8 weeks of recommended unloading time should be doing the trick.
To safely progress loading, we need to know that the fractured bone is healed (via x-ray) and keep the patients pain <2-3/10 during training.
What are normal bone healing times?
In oder to optimally treat fractures, knowing when and how bones heal at their best is of crucial importance.
Fracture healing timeline depends on various factors, such as the presence of comorbid diseases (e.g. diabetes mellitus), surgical technique, trauma severity or patient age.
Bone tissue can heal after being injured (e.g. fracture). But the type of healing (mechanobiology of healing) depends on the fracture fixation technique.
This is due to interfragmentary movements. Meaning, the amount of motion that is enabled to happen between the two (or more) injured bone fragments. One can differentiate between low (= direct conversion of mesenchymal tissue into bone), moderate (=callus healing; where a cartilaginous matrix is converted to bone) and high (=inhibition of bone healing) interfragmentary movement.
To fixate a stable fracture, either splinting or compression will be used.
Splinting can be categorised in:
- External splinting: Such as a plaster cast (mostly for shaft fractures) or an external fixator (when a big soft tissue / open wound is also present) that both allow larger interfragmentary movement and therefore lead to callus healing.
- Internal splinting: via intramedullary nails or internal fixator plates (e.g. for osteoporotic fractures and metaphyseal and epiphyseal fractures).
and compression:
- Compression plates: for metaphyseal and epiphyseal fractures, where direct bone healing can occur without external callus formation.
The distinct phases of fracture healing are mostly based on animal models (from rats). But due to the similarity of the healing processes in humans, this is what we will focus on.
- Inflammatory phase
An inflammatory response is necessary to promote optimal bone healing and can take up to 7 days (in rats). Here, a variety of immune responses are triggered, to prepare the tissue site for repair.
- Repair
The repair phase depends on the fixation technique (primary or secondary bone healing) and the anatomical site of injury (metaphyseal–epiphyseal trabecular bone healing or diaphyseal callus healing). Making the mechanical environment a dominant influence for the type of bone healing.
Primary bone healing (=direct):
Direct bone healing can occur only if interfragmentary movement is minimal and if the two (or more) bone fragments are compressed into each other (via compression plates and lag screws). Here, osteoclastic activity creates tunnels to allow the ingrowth of blood-vessels (angiogenesis). Then osteoblasts create osteons to connect the two fragments. The inflammatory response in this healing type is reduced.
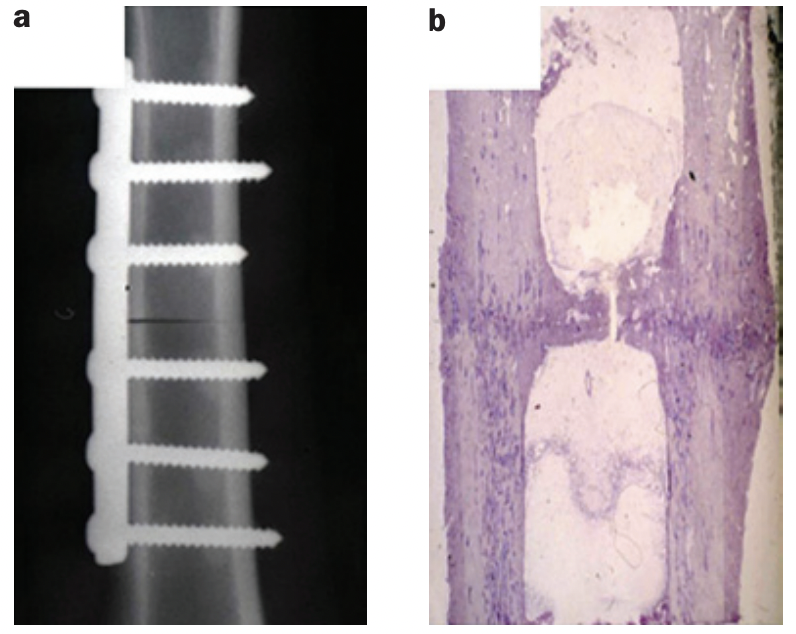
Secondary bone healing (=indirect):
The lower degree of stability of this healing type leads to soft callus formation that ultimately transforms into bony callus. A patient can resume loading of the bone after bony bridging of the peripheral callus has taken place. Which is the sign of successful fracture healing. This can take anywhere form 8-16 weeks (for shaft / diaphyseal bone healing).
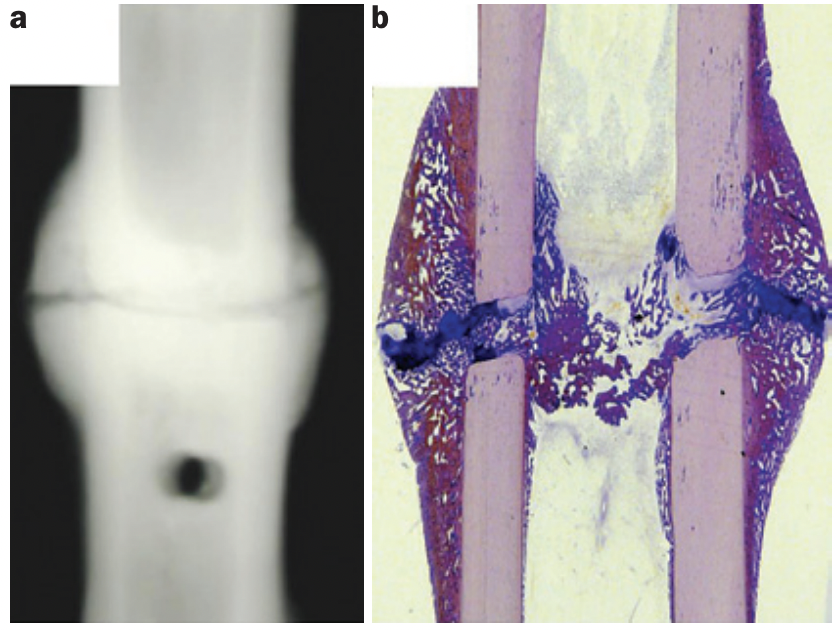
Fracture healing timelines can be delayed due to acute or chronic inflammatory conditions, that can lead to either osteoclastogenesis or to changes in the local (fracture site) inflammatory reaction. Acute inflammation include sepsis or polytrauma, while chronic inflammatory conditions include diseases such as diabetes mellitus or rheumatoid arthritis (Claes et al., 2012).
References:
Barcik, J., & Epari, D. R. (2021). Can Optimizing the Mechanical Environment Deliver a Clinically Significant Reduction in Fracture Healing Time? Biomedicines, 9(6), 691. https://doi.org/10.3390/biomedicines9060691
Bigham‐Sadegh, A., & Oryan, A. (2014). Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. International Wound Journal, 12(3), 238–247. https://doi.org/10.1111/iwj.12231
Bretherton, C. P., Achten, J., Jogarah, V., Petrou, S., Peckham, N., Achana, F., Appelbe, D., Kearney, R., Claireux, H., Bell, P., Griffin, X. L., & WAX Investigators. (2024). Early versus delayed weight-bearing following operatively treated ankle fracture (WAX): A non-inferiority, multicentre, randomised controlled trial. Lancet (London, England), 403(10446), 2787–2797. https://doi.org/10.1016/S0140-6736(24)00710-4
Chandran, M., Akesson, K. E., Javaid, M. K., Harvey, N., Blank, R. D., Brandi, M. L., Chevalley, T., Cinelli, P., Cooper, C., Lems, W., Lyritis, G. P., Makras, P., Paccou, J., Pierroz, D. D., Sosa, M., Thomas, T., Silverman, S., Åkesson, K. E., Blank, R. D., . . . Thomas, T. (2024). Impact of osteoporosis and osteoporosis medications on fracture healing: a narrative review. Osteoporosis International, 35(8), 1337–1358. https://doi.org/10.1007/s00198-024-07059-8
Claes, L., Recknagel, S., & Ignatius, A. (2012). Fracture healing under healthy and inflammatory conditions. Nature Reviews. Rheumatology, 8(3), 133–143. https://doi.org/10.1038/nrrheum.2012.1
Flowers, D. W., McCallister, E., Christopherson, R., & Ware, E. (2022). The Safety and Effectiveness of Early, Progressive Weight Bearing and Implant Choice after Traumatic Lower Extremity Fracture: A Systematic Review. Bioengineering, 9(12), 750. https://doi.org/10.3390/bioengineering9120750
Ganse, B. (2024). Methods to accelerate fracture healing – a narrative review from a clinical perspective. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1384783
Giannoudis, P., Tzioupis, C., Almalki, T. & Buckley, R. (2007). Fracture healing in osteoporotic fractures: Is it really different? Injury, 38(1), S90–S99. https://doi.org/10.1016/j.injury.2007.02.014
Lavoie-Gagne, O., Gong, M. F., Patel, S., Cohn, M. R., Korrapati, A., Forlenza, E. M., Barmonyallah, M., Parvaresh, K. C., Wolfson, T. S., & Forsythe, B. (2021). Traumatic Leg Fractures in UEFA Football Athletes: A Matched-Cohort Analysis of Return to Play, Reinjury, Player Retention, and Performance Outcomes. Orthopaedic Journal of Sports Medicine, 9(9), 23259671211024218. https://doi.org/10.1177/23259671211024218
Palui, R., Durgia, H., Sahoo, J., Naik, D. & Kamalanathan, S. (2022). Timing of osteoporosis therapies following fracture: the current status. Therapeutic Advances in Endocrinology And Metabolism, 13. https://doi.org/10.1177/20420188221112904
Pengelly, J., Boggett, S., Bryant, A., Royse, C., Royse, A., Williams, G. & El-Ansary, D. (2022). SAfety and Feasibility of EArly Resistance Training After Median Sternotomy: The SAFE-ARMS Study. Physical Therapy, 102(7). https://doi.org/10.1093/ptj/pzac056
Pengelly, J. M. S., Royse, A. G., Bryant, A. L., Williams, G. P., Tivendale, L. J., Dettmann, T. J., Canty, D. J., Royse, C. F. & El-Ansary, D. A. (2020). Effects of Supervised Early Resistance Training versus standard care on cognitive recovery following cardiac surgery via median sternotomy (the SEcReT study): protocol for a randomised controlled pilot study. Trials, 21(1). https://doi.org/10.1186/s13063-020-04558-x
Pengelly, J., Pengelly, M., Lin, K., Royse, C., Royse, A., Bryant, A., Williams, G. & El-Ansary, D. (2019). Resistance Training Following Median Sternotomy: A Systematic Review and Meta-Analysis. Heart Lung And Circulation, 28(10), 1549–1559. https://doi.org/10.1016/j.hlc.2019.05.097
Rauschenberg, S., Heinze, V., Schwesig, R., Bethge, S. & Schlitt, A. (2020). Geräteunterstütztes Krafttraining bei aortokoronar bypassoperierten Patienten in der Anschlussheilbehandlung. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin. https://doi.org/10.1055/a-1153-9125
Richardson, C., Bretherton, C. P., Raza, M., Zargaran, A., Eardley, W. G. P., Trompeter, A. J., & FFPOM Collaborators. (2022). The Fragility Fracture Postoperative Mobilisation multicentre audit: The reality of weightbearing practices following operations for lower limb fragility fractures. The Bone & Joint Journal, 104-B(8), 972–979. https://doi.org/10.1302/0301-620X.104B8.BJJ-2022-0074.R1
Robertson, G. A., & Wood, A. M. (2015). Fractures in sport: Optimising their management and outcome. World Journal of Orthopedics, 6(11), 850–863. https://doi.org/10.5312/wjo.v6.i11.850
Saul, D. & Khosla, S. (2022). Fracture Healing in the Setting of Endocrine Diseases, Aging, and Cellular Senescence. Endocrine Reviews, 43(6), 984–1002. https://doi.org/10.1210/endrev/bnac008
Searle, H. K., Lewis, S. R., Coyle, C., Welch, M. & Griffin, X. L. (2023). Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Library, 2023(3). https://doi.org/10.1002/14651858.cd008579.pub4
Singh, S. K., Larkin, K. E., Kadakia, A. R., & Hsu, W. K. (2018). Risk Factors for Reoperation and Performance-Based Outcomes After Operative Fixation of Foot Fractures in the Professional Athlete: A Cross-Sport Analysis. Sports Health: A Multidisciplinary Approach, 10(1), 70–74. https://doi.org/10.1177/1941738117729660
Ulstrup, A. K. (2008). Biomechanical concepts of fracture healing in weight-bearing long bones. 74.
Wang, J., Leung, K., Chow, S. & Cheung, W. (2017). The effect of whole body vibration on fracture healing – a systematic review. European Cells And Materials, 34, 108–127. https://doi.org/10.22203/ecm.v034a08
Warren, J., Sundaram, K., Anis, H., McLaughlin, J., Patterson, B., Higuera, C. A., & Piuzzi, N. S. (2019). The association between weight-bearing status and early complications in hip fractures. European Journal of Orthopaedic Surgery & Traumatology: Orthopedie Traumatologie, 29(7), 1419–1427. https://doi.org/10.1007/s00590-019-02453-z
Weng, S., Bi, C., Gu, S., Qi, X., & Huang, Y. (2020). Immediate weightbearing after intramedullary fixation of extra-articular distal tibial fractures reduces the nonunion rate compared with traditional weight-bearing protocol: A cohort study. International Journal of Surgery (London, England), 76, 132–135. https://doi.org/10.1016/j.ijsu.2020.02.040
Williamson, M., Iliopoulos, E., Jain, A., Ebied, W., & Trompeter, A. (2018). Immediate weight bearing after plate fixation of fractures of the tibial plateau. Injury, 49(10), 1886–1890. https://doi.org/10.1016/j.injury.2018.06.039
Xue, D., Li, F., Chen, G., Yan, S. & Pan, Z. (2014). Do bisphosphonates affect bone healing? A meta-analysis of randomized controlled trials. Journal Of Orthopaedic Surgery And Research, 9(1). https://doi.org/10.1186/1749-799x-9-45
Zhao, K., Dong, S. & Wang, W. (2022). When is the optimum time for the initiation of early rehabilitative exercise on the postoperative functional recovery of peri-ankle fractures? A network meta-analysis. Frontiers in Surgery, 9. https://doi.org/10.3389/fsurg.2022.911471